Automotive Air Pollution (for non-specialist citizens)
- what is smog?
- how did initial industry efforts to reduce pollution only change the color of smog?
- how do automobile manufacturers reduce smog this side of Y2K?
- what is the 'Cole's Notes' version of Dieselgate?
This article is not finished (I have been called away to deal with other more-pressing issues)
Setting the stage
Circa 1965 in North America, passenger automobiles and small trucks were powered by gasoline (petrol for you Brits) which was relatively cheap (45 cents per Imperial gallon in Canada). Buses and large trucks were powered by diesel which usually belched black smoke during acceleration. Since gasoline was cheap, and everyone wanted to go fast, every red-blooded male wanted to own a muscle car. But two were problems looming.
- POLLUTION: There has always been national clean air clean legislation in the USA (here are two examples: 1956
and 1963) but the American nation started thinking differently after Apollo 8 snapped pictures in 1968 of a fragile-looking Earth from lunar orbit. With pressure from both political parties, Richard Nixon created
the EPA (Environmental Protection Agency) on 1970-07-09 with
a mandate to prosecuting environmental laws in order to protect air and water. Nixon also created NOAA (National Oceanic and Atmospheric Administration) in 1970-10-03 after a federal government reorganization.
- PRICE: In 1973, a coalition of Arab nations attacked Israel in what is now known as the Yom Kippur War. The Nixon administration backed Israel which triggered the Arab nations to seek revenge by having OPEC embargo oil exports to North America. That immediately elevated the wholesale barrel price of crude oil from $3 to $12. Since then, the price of oil has skyrocketed and gasoline followed.
Smog (Smoke Fog)
Smog has been around since before the start of the industrial revolution. Yellow Smog was first observed in Great Britain and was usually associated with burning high-sulfur coal (eg. Lignite is one example). I mention this here because non-technical people reported seeing yellow smog in California during the late 1960s when virtually no one was burning coal, but every family owned multiple cars. Also at that time, people reported seeing both brown and gray smog.
Scientists and engineers know that the color of NO2 (nitrogen dioxide) can be perceived as reddish-orange to reddish-brown so now we have a possible explanation for the brown smog. In the presence of water, NO2 can be transformed to HN03 (nitric acid) which is pale yellow, and this is a possible explanation for yellow smog seen in California.
Grey smog is usually associated with soot produced by burning coal (or wood) but since California is a warmish state, engineers assumed this was unburned hydrocarbons from cars (gasoline) or buses and trunks (diesel). Since fuel was becoming expensive and pollution was in the rise, automotive engineers decided it was time to make vehicles more fuel efficient.
Improving Engine Efficiency
The most efficient fossil-fuel powered engine only releases carbon dioxide (CO2) and water (H2O). Energy is lost whenever these engines release unburned hydrocarbons (CHx), carbon monoxide (CO) or soot (C). Solutions include:- increasing the oxygen/fuel ratio
- Carburetors
- Fifty-years ago, many vehicles employed a carburetor to mix fuel with atmospheric air before presenting it to a combustion engine. A carburetor is nothing more than a glorified perfume atomizer where the starting mixture is controlled by turning a screw (rich vs lean). In 2021 my property maintenance machines (snow blower, lawn mower, weed-wacker) still employ carburetors.
- A simple carburetor tuned to work efficiently at sea-level might not behave properly when you drive to a mountainous area where atmospheric air pressure is lower, so mechanical feedback mechanisms were introduced to add-to, or subtract-from, the starting setting.
- Supercharger (and turbocharger)
- Things were different in the aircraft industry where huge changes in pressure and temperature made carburetor use unreliable. So many non-jet aircraft designers employed superchargers to ensure that sufficient air and fuel were always available. Most people today seem to be unaware of the fact that superchargers predate the invention of powered flight and horseless carriages.
- Initially, superchargers were only available on expensive most racing equipment as well as expensive European autos but as time moved forward they began to appear in the consumer space.
- Alert: a supercharger "is a pump which requires power" which, almost always, comes from the engine via a crankshaft belt. Superchargers "powered by engine exhaust" go by the name "turbocharger"
- Carburetors
- improving fuel evaporation from a liquid to a gas
- according to Boyle's law, a supercharger (or turbocharger) will cause fuel droplets to evaporate as soon as the pressure is increased.
- some solutions preheat the air-fuel mixture as it passes through the intake manifold
- improving engine design
- Wikipedia's article titled Chrysler's hemispherical engine is good so I won't repeat that content here
Details about burning Gasoline ('petrol' for you Brits)
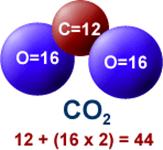 At first glance it seems impossible (to a non-scientist)
that:
At first glance it seems impossible (to a non-scientist)
that:
which weighs approximately 6 pounds (2.7 Kg)
will produce produce produce 18 pounds (8.16 Kg) of carbon dioxide (CO2)
Why? While the "Carbon" in CO2 does come from the fuel, the Oxygen comes from the atmosphere which is usually never considered by the non-specialist.
DETAILS: When gasoline burns, the Carbon and Hydrogen separate (which releases energy in the form of heat). The Hydrogen combines with Oxygen (from the atmosphere) to form water vapor (H2O) while the Carbon combines with Oxygen (from the atmosphere) to form carbon dioxide (CO2). A carbon atom has an atomic weight of 12, and each oxygen atom has an atomic weight of 16, giving each single molecule of CO2 an atomic weight of 44 (12 + 2 x 16 ).
COMMENT: It now appears that that Carbon Capture and Storage (CSS) technology will never be practical since the required amount of energy to compress-store this volume
of gas would be too great.
- The players in this drama
Substance Chemical Formula Notes Molecular Oxygen O2
Octane (gasoline or petrol) C8H18
Carbon Dioxide CO2
Water H2O
Molecular Nitrogen N2 An inert gas at room temperature
Can bond with O to produce yellow smog - Atomic Masses from the Periodic Table:
Element Atomic Number Atomic Mass Hydrogen 1 1 Carbon 6 12 Oxygen 8 16 - (balanced) Burn Equation: 2 C8H18 + 25 O2 → 16 CO2 + 18 H2O (reference)
- Gasoline Mass Calculation
- Total octane mass (from the left-hand side of the equation):
- 2 x ((C x 8) + (H x 18))
- 2 x ((12 x 8) + (1 x 18))
- 2 x (96 + 18)
- 2 x 114 = 228
- Total Oxygen Mass (from the left-hand side of the equation):
- 25 x (O x 2)
- 25 x (16 x 2)
- 25 x 32 = 800
- Total octane mass (from the left-hand side of the equation):
- Carbon Dioxide Mass Calculation
- Total Carbon Dioxide mass (from the right-hand side equation):
- 16 x ((C x 1) + (O x 2))
- 16 x ((12 x 1) + (16 x 2))
- 16 x (12 + 32)
- 16 x 44 = 704
- Ratio: 704 / 228 = 3.09 (therefore the resultant CO2 is ~ 3 times heavier than gasoline)
- Total Carbon Dioxide mass (from the right-hand side equation):
- Water Vapor Mass Calculation
- Total Water Vapor mass (from the right-hand side equation):
- 18 x ((H x 2) + (O x 1))
- 18 x ((1 x 2) + (16 x 1))
- 18 x (2 + 16)
- 18 x 18 = 324
- 18 x ((H x 2) + (O x 1))
- Ratio: 324 / 228 = 1.42 (therefore the resultant water vapor is ~ 1.4 times heavier than gasoline)
- Total Water Vapor mass (from the right-hand side equation):
The Diesel Scandal (article scaffolding; this is not complete)
- diesel engines are built to higher tolerances because they have much higher compression ratios
- the compression ratio is so high that most engines do not require a spark plug
- as each piston finishes "the compression stroke" the temperature rises (Gay-Lussas's Law) causing the fuel to automatically ignite
- some systems employ glow-plugs to to help start the engine on very cold days, or remote locations like the the north and south poles
- Atmospheric Nitrogen is usually inert. But at the very high temperatures resulting from higher compression ratios, combined with very lean fuel (read as: too much
oxygen in order to enure that 100% of the fuel is burned), Diesel engines to convert atmospheric (diatomic) nitrogen (N2) into pollutants like these few of many:
NO Nitrogen Monoxide NO2
Nitrogen Dioxide
HNO3
Nitric acid
- In order to limit the production of these pollutants, diesel engines employ a chemical product known as AdBlue® which is injected into the exhaust system.
- When the chemical runs low, a blue light (or LED) is illuminated on the dash telling the owner to visit the dealer (many people buy the chemical at a big box store then fill it up themselves)
- Now the story gets weird:
- Volkswagen upper management wanted to sell more diesel vehicles around the world as a greener alternative to gasoline engines; but the consumer grumbling about AdBlue along with some complaints that some drivers need to refill the AdBlue reservoir 3-4 times a year appeared to be limiting sales
- Without consulting their engineers, management announced that Volkswagen was going to produce an diesel engine that did not require AdBlue. Rumor has it that they spent $100 million before giving up (if asked ahead of time, the engineers would have told management that what was being proposed was impossible)
- So after spending a huge sum of money with nothing to show for it, Volkswagen began selling diesel products which:
- did not employ AdBlue
- claimed that Volkswagen diesel engines were cleaner, when in fact, they were actually dirtier.
- contained a software patch to the engine software (also called firmware) that could detect when the engine was being environmentally tested. At that time only, the system would alter engine parameters in such a way as to reduce engine performance (including temperature) which would reduce the production of nitrogen pollutants.
- IMHO Volkswagen management was very naive because their competitors were also looking for solutions to eliminate AdBlue so would, most likely, be looking for a way to copy (or cross-license) Volkswagen's solution to this problem.
Links
- https://en.wikipedia.org/wiki/Volkswagen_emissions_scandal
- introduction to heat pumps
- introduction to climate modeling
- http://www.severe-weather.eu/
 Back to Home
Back to HomeNeil Rieck
Waterloo, Ontario, Canada.